
Parkview Laboratories is replacing the existing BD AFFIRM vaginitis panel with the BD MAX+ vaginitis panel. Go-live for this test is 6/17/24 at 0700.
The existing/old method will only be accepted on a limited basis through 6/19/24, and testing will be rejected on later dates.
There will be a slight delay in resulting the old method after 6/17/24 0700 due to the switch. The test will continue to only be performed at PRMC.
The sensitivity and specificity of this test is much improved and allows for faster turnaround on test results. Providers will receive Detected/Not Detected results for the targets below:
-Bacterial Vaginosis algorithm
-Candida group (C. albicans, tropicalis, parapsilosis, dubliniensis)
-Candida krusei
-Candida glabrata
-Trichomonas vaginalis
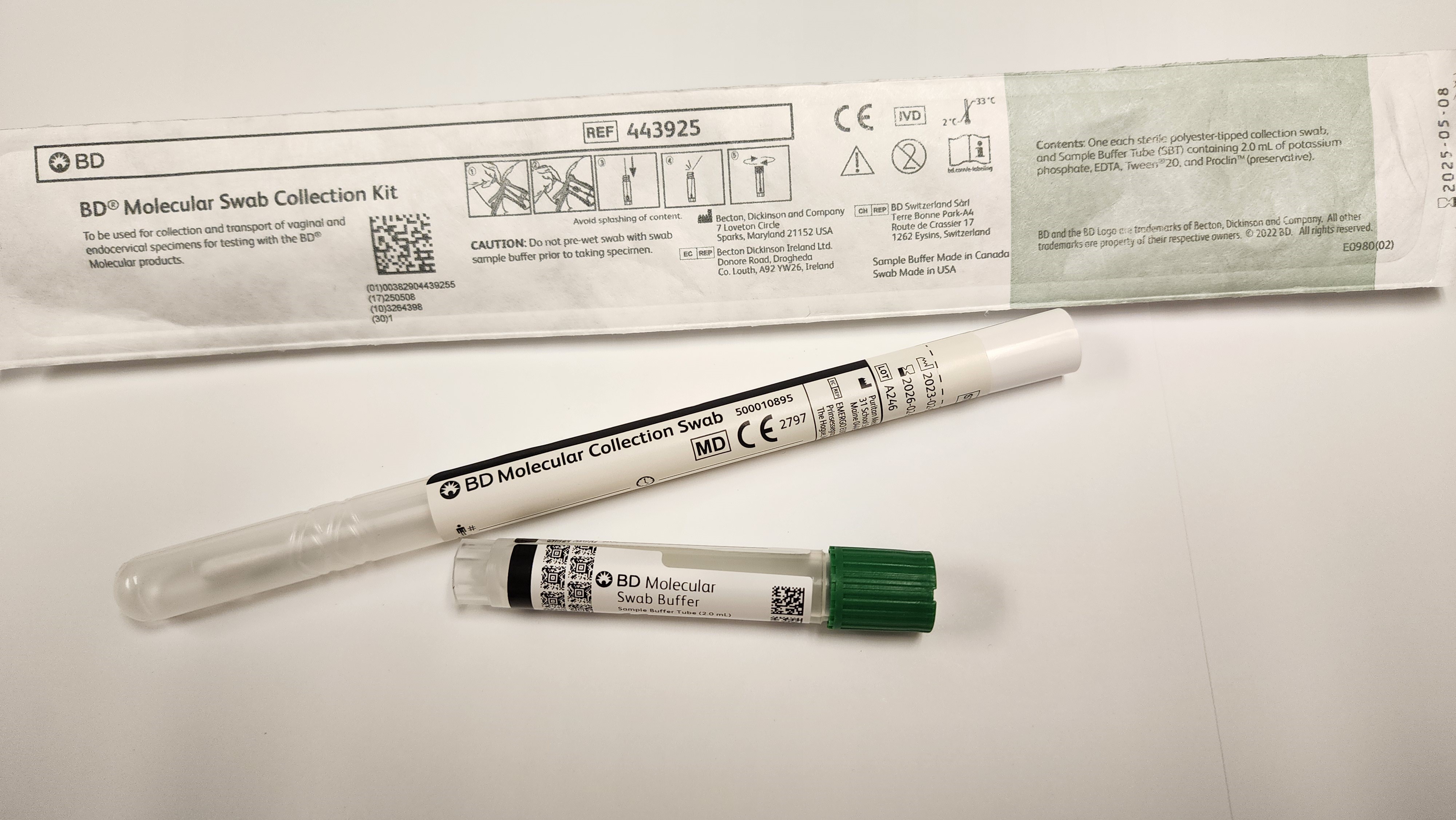
The new test requires a new collection swab/device, as seen at the left.
This may be collected by providers AND patient self-collect in-office. For these instructions and more information please click here
Swabs are not to be sent home with patients.
No lubricants may be used before/during collection of this test.
Labels should applied along the length of the green capped tube as below, leaving some of the square barcodes visible. See Sample to the left
For more information contact Stephania Turkette, Microbiology Specialist.
Stephania.turkette@parkview.com
260-266-2583